Term Used to Describe Force Holding Molecules Together
And number two we are looking for inter molecular forces. If youre seeing this message it means were having trouble loading external resources on our website.

Interintramolcforces Gif 500 330 Teaching Chemistry High School Chemistry Ap Chemistry
Describe the intermolecular forces that hold bromine molecules together in the liquid state between the temperatures of 72C and 588C and in the solid state below 72C.

. Induced dipole forces A great example of hydrogen bonding holding molecules together to. Introduction to Chemistry 4th Edition Edit edition Solutions for Chapter 10 Problem 140AQ. The truth is quite complicated to explain here but I.
See also what does coral eat. This is the term to describe the attraction between one nonpolar molecule and another nonpolar molecule. All of the attractive forces between neutral atoms and molecules are known as van der Waals forces although they are usually referred to more informally as intermolecular attraction.
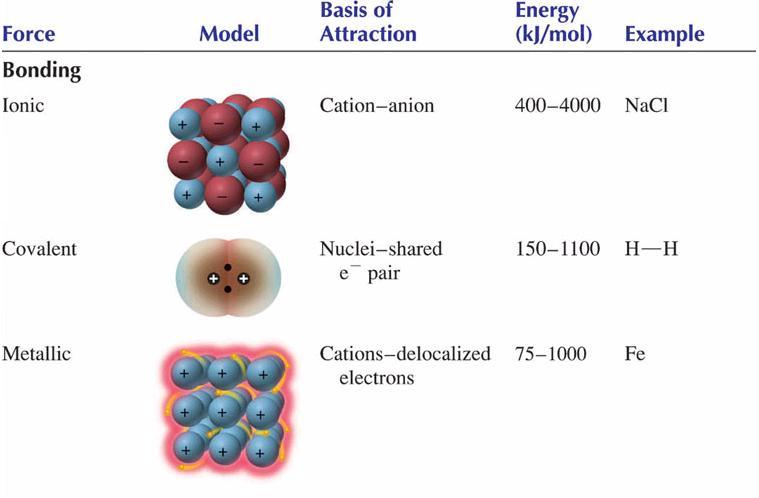
Bonding both ionic and covalent provides a description of the amazing ability of solids to hold themselves together. May 19 2006. A force holding two atoms together is an chemical bond.
Its a correct answer. Two things cause a gas to deviate from the ideal - attractive forces from other molecules and the size of the molecules effect on the number of collisions. So what that results in is covalin bonding.
But Id like to understand and explain the force that makes this work. This is the force inside a molecule of bromine hold the molecule together. A chemical bond is an electrical force linking atoms.
Even the rule that atoms need to try to be surrounded by a complete shell of electrons is just a part of the description that works well for chemistry. All of the attractive forces between neutral atoms and molecules are known as van der Waals forces although they are usually referred to more informally as intermolecular attraction. These forces control the movement of molecules and atoms.
J The overall change in the molecule caused by the introduction of a carbon-carbon double bond is the bent shape it gives which results in atoms not fitting as close together when they were just single bonds originally. In any solid the particles have bonds holding the atoms together into molecules and bonds or forces holding the molecules together to. Both types of forces determine the chemical and physical characteristics of substances.
Dispersion forces are however the only intermolecular force experienced by. So I am forces are forces between molecules that hold molecules together not the forces. A chemical bond is the force that holds the atoms of a molecules together as in a compound.
London Dispersion and hydrogen bond charges are the intermolecular forces holding the molecules together 8. The forces that hold the _____ together in compounds and molecular elements are called chemical _____. The water molecule has two distinct ends each with.
Thats pretty much the main factor. Match the term concerning hydrocarbon in the left column with the correct description in the right column. A chemical bond formed by the sharing of one or more pairs of electrons between the outer shells of two atoms is called an _____ bond.
Chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. Low pressure basically removes the attractive force bc there are too few molecules close enough to affect the other. Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion forces.
This is quite an answer and not accurate. Intermolecular and intramolecular forces are the two types of forces that hold individual molecules and atoms together. The following points highlight the five main forces that stabilise protein structures.
Water H2O is a covalent compound because the bond forms between two hydrogens and one oxygen are covalent in nature. Select all the statements that. Chemical bonds hold molecules together and create temporary connections that are essential to life.
Answer 1 of 9. Two idealized types of bonding are ionic bonding in which positively and negatively charged ions are held together by electrostatic forces and covalent bonding in which electron pairs are. Scientists will tell you that the particules in the nucleus proton and neutron mainly proton with their positive charge are held together by what they called the strong force.
Intermolecular forces hold multiple molecules together and determine many of a substances properties. The covalent bond is formed due to the sharing of electron occurs between hydrogen and oxygen atoms in order to complete their octet shell and hence attains stability. Van der Waals Forces.
The SNF is responsible for holding the protons in a nucleus together against the force of their electrostatic repulsion due to having like charges. This is the force between two molecules of bromine holds the molecules together Dipole dipole. The main difference between intermolecular and intramolecular forces is that intermolecular.
Dispersion forces are experienced by ALL particles atoms ions and molecules. Intermolecular forces hold multiple molecules together and determine many of a substances properties.

Intermolecular Force Easy Science Intermolecular Force Force Definition Study Skills

Helium Adalah Suatu Unsur Kimia Dalam Tabel Periodik Yang Memiliki Lambang He Dan Nomor Atom 2 Pinterpandai Atom Atomic Theory Physics
No comments for "Term Used to Describe Force Holding Molecules Together"
Post a Comment